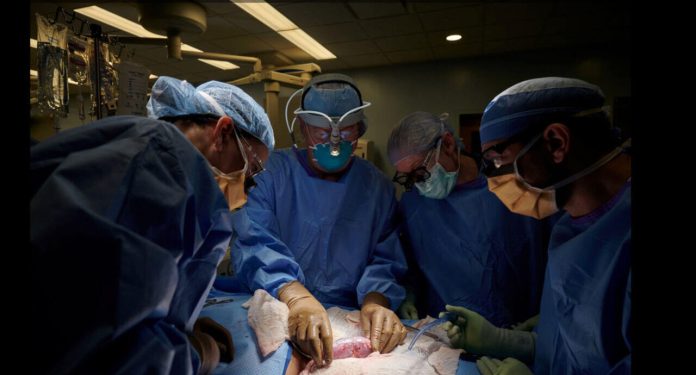
A pig kidney has been transplanted into a person for the first time without the recipient’s immune system rejecting it right away, a potentially momentous breakthrough that could eventually help ease a critical shortage of human organs for transplant.
The treatment, which took place at NYU Langone Health in New York City, used a pig whose genes had been altered so that its tissues no longer carried a chemical that causes a virtually immediate rejection.
The receiver, according to Reuters, was a brain-dead patient with kidney disease whose family agreed to the experiment before he was taken off life support.
The new kidney was linked to his blood veins and placed outside his body for three days, allowing researchers to examine it.
The transplanted kidney function test results “appeared fairly typical,” according to transplant surgeon Dr. Robert Montgomery, who conducted the study.
When the undigested pig kidney was implanted into non-human primates, the kidney generated “the amount of pee you would anticipate” from a donated human kidney, he said, and there was no evidence of vigorous, early rejection.
The recipient’s elevated creatinine level, which is an indicator of impaired kidney function, reverted to normal following the transplant, according to Montgomery.
According to the United Network for Organ Sharing, roughly 107,000 people in the United States are waiting for organ transplants, with more than 90,000 waiting for a kidney. A kidney takes three to five years to mature.
Researchers have been working on the potential of using animal organs for transplants for decades, but they haven’t figured out how to prevent the human body from rejecting them right away.
Montgomery’s team hypothesized that removing the pig’s gene for a carbohydrate that causes rejection — an alpha-gal sugar molecule or glycan — would solve the problem.
United Therapeutics Corp’s (UTHR.O) Revivicor Unit created the genetically engineered pig known as GalSafe. The US Meal and Drug Administration approved it in December 2020 for use as a food for persons with meat allergies as well as a possible source of human medicines.
Medical items created in pigs, according to the FDA, would still require particular FDA permission before being utilized in people.
Other researchers are looking into whether GalSafe pigs may be used to make everything from human heart valves to skin grafts.
According to Montgomery, who received a heart transplant himself, the NYU kidney transplant experiment should pave the way for studies in patients with end-stage kidney failure in the next year or two. The technique might be used as a temporary remedy for critically ill patients until a human kidney becomes available, or as a permanent graft in such studies.
Montgomery explained that because the present trial had only a single kidney transplant and the kidney was only left in place for three days, any future trials are likely to find new challenges that must be overcome. Patients with a low chance of acquiring a human kidney and a bad prognosis on dialysis are likely to participate.
“When it comes to them, the death rate is as high as it is for some malignancies, and we won’t hesitate to use new treatments and perform new testing [with cancer patients].”
Montgomery stated, “A few months can give you more life.”
Before asking a family for brief access to a brain-dead patient, Montgomery said the researchers worked with medical ethicists, legal, and religious experts to investigate the concept.

















![10 Countries With the Best Healthcare in the World [Statistical Analysis] Countries With the Best Healthcare in the World](https://articleify.com/wp-content/uploads/2025/07/Countries-With-the-Best-Healthcare-in-the-World-1-150x150.jpg)









