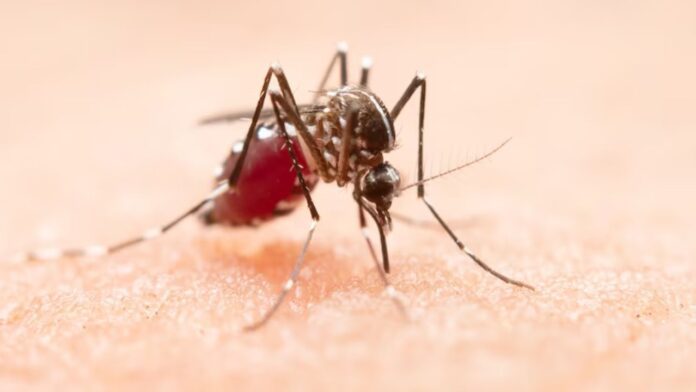
The FDA has granted approval for the first vaccine against chikungunya, a mosquito-transmitted virus that causes high fever and severe joint pain. The vaccine was developed by the European pharmaceutical company Valneva.
While chikungunya is rarely fatal, it can cause debilitating symptoms that last for months or years. The disease primarily occurs in tropical and subtropical regions of Africa, Asia, and the Americas. In recent years, localized transmission has been documented in Europe as well.
The newly approved single-dose vaccine is authorized for use by adults ages 18 and older. Its approval in the U.S. is expected to accelerate global access to immunization against this emerging public health threat.
What is the Chikungunya virus?
Chikungunya virus is spread through the bite of an infected mosquito, mainly Aedes aegypti and Aedes albopictus species. Common symptoms include high fever, joint swelling and pain, headache, muscle pain, and rash. While many patients feel better within a week, some experience recurrent symptoms for months or years.
Newborns infected around the time of birth are at risk for severe, life-threatening illness. There are currently no vaccines or specific treatments available for chikungunya. Avoiding mosquito bites is the only way to prevent infection. In addition, you can also read an article on- Lingering Cold and Respiratory Virus Symptoms: A Hidden Epidemic?
The virus has spread rapidly in recent years.
First identified in Tanzania in 1952, major outbreaks of chikungunya occurred in countries in Africa and Asia through the 1960s and 1980s. Starting in 2004, the virus caused large epidemics on islands in the Indian Ocean, India, and parts of Southeast Asia.
Local transmission was reported for the first time in Europe in 2007 and in the Americas in 2013. As of 2022, over 100 countries have reported evidence of chikungunya virus infection. At least 5 million cases have occurred since 2006.
The New Vaccine Could Help Curb Ongoing Outbreaks
Valneva’s vaccine, named VLA1553, is the only chikungunya vaccine to receive FDA approval to date. In clinical trials, it was highly immunogenic and well-tolerated in adults. Safety and efficacy were demonstrated in a pivotal Phase 3 trial including over 4,000 participants.
“This new vaccine could help curb ongoing outbreaks and prevent future ones,” said an FDA representative. Widespread vaccination may limit the spread of the virus, particularly in high-risk areas struggling with recurrent epidemics.
In addition to FDA approval, Valneva has applied for authorization with the European Medicines Agency. The company expects to make the vaccine available for purchase in the U.S. later in 2023.
You May Find Interest: Nipah Virus: Everything You Need to Know [Latest Health Tips]

















![10 Countries With the Best Healthcare in the World [Statistical Analysis] Countries With the Best Healthcare in the World](https://articleify.com/wp-content/uploads/2025/07/Countries-With-the-Best-Healthcare-in-the-World-1-150x150.jpg)









